Freedom. Choice. The ability and right to make informed decisions. These are concepts deeply valued by most people around the world. Sadly, the value of these ideals has not been shown to the tube-fed community. They are ideals the tube-fed community has had to fight for for years.
Hospitals are still forcing parents to feed their tube-fed child formulas that make them vomit. Medical professionals are calling child protective services on parents for no other reason than the parent feeding their child food. Freedom? Choice? Nope.
Parents and patients are being given entirely inaccurate information about the health outcomes associated with the use of enteral formula, removing their ability to make informed decisions. It is happening every single day. But, this is not only happening when it comes to what is being fed through a feeding tube.
ENFit® products for feeding tubes are another example of this occurring. I’ll be clear from the beginning. I am not against people using ENFit® at all. However, I am absolutely against the approach that has been taken with the creation and implementation of ENFit® as it has violated the concepts of freedom, choice, and the ability to make informed decisions from day one for the tube-fed community.
What prompted the development of ENFit® ?
In 2006, a pregnant woman named Robin Rodgers was in the hospital, and someone took the line coming from her IV and spiked it into a feeding pump bag filled with enteral formula. This error resulted in enteral formula being fed directly into her veins. Soon after, both she and her baby passed away.
It was a tragedy, and it should never have happened. In fact, injuries and fatalities from these kinds of misconnections have been reported as far back as 1972. Though rare (24 reported incidents over the course of 7 years), they should be non-existent, given how avoidable these incidents are.
And so, the decision was made to develop a new system for tube feeding so that enteral feeding devices could never be connected with non-enteral devices. And all of this is fine and well.
That is how ENFit® came into existence.
The issue with ENFit® is that it introduces safety issues of its own, and tube-fed people and their caregivers should be aware of these safety issues so they can decide whether ENFit® is right for them. However, that is not what is happening. Further, efforts are being made to force all tube-fed people to use ENFit® products even if they do not wish to do so. This will never be acceptable.
Before I get into more information, let’s think about this. Imagine if orally-fed people were suddenly forced to use chopsticks instead of forks and spoons even if they preferred to use forks and spoons. There would be an outrage. This wouldn’t mean there aren’t situations where chopsticks are a better fit for a person or family. But, we all want freedom to choose. Having a feeding tube shouldn’t change that.
So, what are the safety issues associated with ENFit® ? I’ve spent quite some time looking all of this over, and the following seem to be the primary safety issues.
The bacterial load in the deep moat area of the ENFit® patient side connector is of serious concern.
This is the biggest safety issue, from what I’ve seen, associated with ENFit®. Nelson Report data shows that even when brushed, the moat area in the male side of the connector has a bioburden too numerous to count. This means there was so much bacteria in it, there was more than could even be counted.
Alarmingly, rather than people being told about this 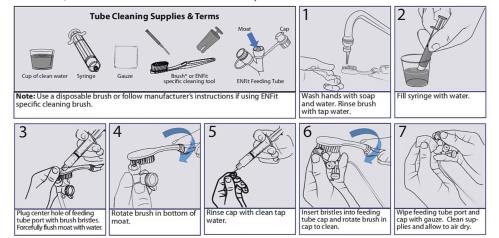 issue, ENFit® forged ahead while users remained unaware that this is even a problem. Many who later discovered this information made repeated complaints, which is when patients were advised to clean the connector with a toothbrush or other brushes (the picture above was taken from stayconnected.org).
issue, ENFit® forged ahead while users remained unaware that this is even a problem. Many who later discovered this information made repeated complaints, which is when patients were advised to clean the connector with a toothbrush or other brushes (the picture above was taken from stayconnected.org).
There are multiple problems with this. First, the moat area has variable depths, depending on the manufacturer. Will the brush reach deeply into all of them? No one has ever checked. Secondly, it’s questionable if this is safe in a hospital setting. A moving brush means spatter of bacteria into the air and onto surfaces.
Third, and most significantly, this method has not been tested to show it even works. Users should be asking, “Where is the safety data related to bacteria in the moat area and this method of cleaning?”
It seems that the patient side connector should always be smooth, without ridges, and without a moat. Were the developers willing to try something else upon this discovery of potentially dangerous contamination? Apparently not.
None of this is to say no one should use ENFit®. However, this is information every user should know about and those who do use ENFit® should use great care in cleaning the moat area.
The point of disconnect when the tube is accidentally yanked or pulled is moved to the actual feeding tube itself.
The screw thread locking system of ENFit® is both a blessing and a curse, particularly for those who are fed via a feeding pump. I myself experienced plenty of episodes of “feeding the bed” and having my child miss out on meals due to disconnections that went unnoticed. While for my child, this resulted in little more than an inconvenience of washing bed sheets and such, for other people, this can be extremely dangerous.
There are individuals that depend on steady tube feeding throughout the night to manage blood sugar levels, and a disconnection can lead to terrible consequences. It should be noted, however, that there is a product designed to address this problem (the AMT Medical Inc. tube clamp).
On the other hand, ENFit® doesn’t eliminate the risk of disconnection. It simply changes the weak point at which a tube feeding system can become disconnected from a person. With ENFit®, that weak point becomes the feeding tube itself. With ENFit®, when a feeding delivery set is accidentally caught or yanked, rather than it disconnecting at the juncture between the feeding set and extension, the force can inadvertently remove the entire feeding tube from the individual.
This can be especially problematic for those fed via a J-tube as they cannot be replaced at home and require a trip to the hospital. Even g-tube users, however, could be injured with a full balloon getting yanked through a stoma.
I have heard from a family with a mother who is J-tube dependent. They were quite worried about being forced to eventually switch to ENFit® as her small children routinely created situations where her tube was disconnected. With a break-free point where the delivery set connects to the extension, this family managed this quite well. This point would disconnect, and they would simply reconnect it when that occurred. However, they are fearful if they are forced to use ENFit® , their situation will become much more complicated as her j-tube could easily be forced out.
All of this is not to say no one should use ENFit®. Parents with wiggly children have often reported strongly in favor of ENFit®. But, as you can see, it’s not ideal for all situations, and this is a benefit/risk ratio that every family should be aware of and have the ability to choose for themselves which system will work best for them. The benefit/risk ratio will vary from family to family and individual to individual.
Some viscous and crushed medications as well as some blenderized food cannot fit through the smaller opening.
ENFit® products have a much smaller flow path than the previous feeding tube systems, which can be problematic for some people. Some medications simply cannot fit through the smaller opening. If a person cannot swallow at all, which many tube-fed people cannot, how else would they take their medication?
Likewise, I know of many children who require a very thick blenderized diet. I have heard from some families who have struggled greatly with ENFit® due to the smaller opening and their love one’s particular needs. What would these people do without access to other systems? In spite of this, efforts have continued to push medical suppliers and hospitals to provide only ENFit® products for tube feeding.
People with specific disabilities and illnesses cannot independently use ENFit® .
Because of the screw thread lock system, some people with specific disabilities are unable to use ENFit® products without assistance while they can use non-ENFit® products entirely independently.
I have a friend who is known online as The Traveling Tubie. He is a disabled veteran and cancer survivor who is dependent on a feeding tube and lives with one arm. In my book, Stand for Food, he wrote a section in the appendix about a dark time in his life in which having a feeding tube took away his independence because he required the assistance of his wife to be fed.
A member of the adult tube-fed community shared a device with my friend that granted him the ability to feed himself on his own in spite of living with only one arm. He found freedom once again and now had opportunities to take classes and do many more productive activities with his life. A forced switch to ENFit® would take all of this away from him. And he’s not alone.
There are other tube dependent people with only one functional arm. There are also many tube dependent individuals living with arthritis or other issues that impact their fine motor skills and dexterity who would find the ENFit® system impossible to use on their own. In spite of this, efforts have continued to push medical suppliers to provide only ENFit® tube feeding systems.
ENFit® products can wrongly connect with another device that would be just as dangerous as connecting to an IV
I mean, this is just ridiculously ironic. The whole point of ENFit® was to avoid dangerous and potentially fatal misconnections of enteral feeding devices to non-enteral devices. Yet, an ENFit® feeding syringe or pump set can fit right into an adult tracheotomy airway. Obviously, sending pureed food or formula through a trach into a person’s lungs would be devastating to the recipient.
While one could argue that it is absurd to imagine someone connecting an enteral feeding device to a trach, is it any more absurd than the notion of someone managing to shove an enteral feeding device into an IV (something that is not possible even if someone tried)?
If the whole reason ENFit® was made was to avoid dangerous misconnections, it defeats the primary purpose if it can be connected to other kinds of tubes that would be just as dangerous. All this made me wonder whether the development of ENFit® was truly aimed at patient safety or whether other motives were at work. And this leads me to my final point.
The process through which ENFit® was developed was very shady and showed very little, if any, consideration for tube-fed people.
I have multiple issues with the process through which ENFit® was developed. It was shady from the start. An organization called GEDSA, which is a non-profit organization, was assembled to develop a tube feeding system that resolved the safety concerns mentioned earlier.
Its original executive director was a guy named Tom Hancock. Prior to becoming the executive director at GEDSA, he was the director of marketing for both therapeutic nutrition and pediatric products for Abbott Nutrition.
All of the poorly done research by Abbott Nutrition that has been used to frighten the medical profession away from blenderized diets (you can learn more about this here, here, and here)…all of the ridiculous claims about how great Pediasure supposedly is in spite of a total lack of valid data to support such a claim…kids being fed over 100 grams of sugar daily in products Abbott describes as nutritious…all of the kids vomiting due to their atrocious products…
This guy was behind a large amount of the marketing that lead to this stuff happening.
I want to make this very clear to show what kind of a person Tom Hancock is. Pediasure is marketed by Abbott Nutrition as a wonderful product that helps kids grow. This is marketed to parents with children who have access to a wide variety of food, and these parents are told there is clinical data to support the claim that their child, who has access to enough food, will grow better if they are fed Pediasure. Remember that while thinking about the studies they cite to support this.
These are the studies they reference:
1. A study on children in Pakistan who do not have access to enough food to consume an adequate amount of calories. In other words, the children did not have access to enough food and were starving. They were given Pediasure, and grew and gained better than they did when they were starving. What a shocker…give a kid enough calories and they will grow better than when they don’t have access to enough calories.
None of this says anything positive about Pediasure specifically. It simply means children will starve if they don’t have access to enough food. This study could have gotten the exact same results by giving the children sandwiches to increase their caloric intake. Yet, this study is used to push parents to feed their child Pediasure even though their child is not starving and does have access to adequate food.
2. Another study claims that kids who are “picky eaters” grew better and had less infections when fed Pediasure than when they were not fed Pediasure and simply offered nutritional counseling. But, they leave out some important details about this study. This study took place in Taiwan and the Philippines, countries where poverty-related malnourishment is extremely common.
The way they determined who was a “picky eater” was completely arbitrary, not well-defined, and at the discretion of the investigators…investigators meaning employees of Abbott Nutrition, the makers of Pediasure.
This is not how science is done. If “picky eaters” are going to be used for a study, a method of defining picky eating that is specific and quantitative should be designed first to determine who would qualify to participate in the study and who would not. Science absolutely requires this. A clear part of this criteria would be children who have access to an abundance of food, but are not eating it.
But, that isn’t what they did. They left it to the discretion of the investigators. That alone makes this a very unscientific study. They used no clearly defined measure to determine who is a true picky eater as opposed to a child who simply doesn’t have access to enough food to insure the children in their study were actually picky eaters rather than children who were starving.
They had the parents list what their child had eaten over the prior 3 days, and if it was not enough food, they labeled the child a picky eater. Well, what if the family simply didn’t have any other food to offer their child?
Why go all the way to the Philippines for a study like this? Abbott Nutrition is located just north of Chicago. Why not find some kids who have plenty of food in their home and are not eating enough right in their area? Probably because they know they wouldn’t get the results they were looking for. Notice in the abstract, they did not even mention what countries these studies were conducted in, which is unusual for a scientific study done in more poverty-stricken areas of the world.
They needed to go, like in their first study, to an area where children were starving. Of course, nutritional counseling won’t help a family if the family does not have access to enough food in the first place. Of course, children being fed Pediasure will do better than children who are literally starving.
Why not provide a control group where the families are provided with enough food for their children and compare how those children do with those who are simply fed Pediasure? Abbott is not interested in a study like this because they know they likely wouldn’t get results that they can twist around to make their products sound nutritious and beneficial to health.
Do I need to go on? I could. They purposely go out of their way to do crappy research and then, twist around the data collected from this crappy research to deceive parents into using these products through false and unproven claims. This is actually illegal in the United States as well as many other countries. And it is unethical in all countries.
This Tom Hancock guy…he was in on this. He helped Abbott Nutrition do this. He was in charge of this bullshit for years while these products made many children violently ill and Abbott laughed their way to the bank.
This is the guy who was put in charge of all things ENFit® . Let that sink in. Is this really someone we can trust to use data properly to develop a feeding system for ourselves or our loved ones?
When ENFit® was developed, by their own admission, whether or not pureed food could pass through the system was never something they took into consideration in spite of knowing how many tube-fed people depend on pureed food to survive.
Was ENFit® truly developed with patient safety in mind? Does Tom Hancock have a history of showing concern for patient safety? No.
Hancock ran GEDSA, funded by donations, to develop ENFit® and used the organization to push to make other feeding devices illegal or ineligible for reimbursement from Medicare and Medicaid. As all this was going on, he was in the process of founding Vesco Medical, a company that would make the products developed through these donations and, if he had his way, would be mandated to everyone around the world, whether it worked for their situation or not.
Then, he left GEDSA and now runs a company that makes these very products. Suspicious? Indeed.
Here is what we know. Adequate testing of the cleanliness of the moat area was not conducted in the development of this product. Shouldn’t this be a primary concern when developing a product used for eating and drinking? Even when it was shown this area has an unacceptably high bacterial load, the pushing of ENFit® forward continued full steam ahead.
We know that this system was shown to be extremely unsafe for use in the NICU (you can learn about this here)…and in spite of it, the development of this system still went full steam ahead. I talked to the new executive director of GEDSA on the phone about this issue, and he said it was determined that the issue with ENFit® that could cause a premature baby to overdose was found to be resolved by tapping the syringe on a hard surface to clear that area where extra medication could build up.
What? I had to express my concern to him being the mother of a child who was born extremely premature. Extremely premature babies have little to no immunity. Any infectious illness, no matter how mild to the rest of us, can be fatal to a baby that weighs one pound.
So much of life in a NICU centers around keeping things sterile as much as is possible while still having people in it. My hands and arms were covered in rashes from the numerous times I had to scrub them throughout each and every day to protect my son. Tapping a syringe on a surface before hooking it up to his feeding tube increases the risk of giving a premature baby an infection. This is not a solution.
Why are we developing protocols for medical devices that involve things like banging the device on a counter top and tooth brushes?
We know that the joint commission refused to endorse ENFit® . That is significant.
We know the needs of many tube-fed people were largely ignored during its development.
Lastly, this entire process of developing ENFit® has used Robin, the woman who lost her life due to a misconnection, as a poster child for their work. That’s a bit odd considering the point of misconnection that resulted in her death was not even at a point that ENFit® addresses in any way. Her IV was connected to IV tubing just fine. A feeding tube delivery set cannot be shoved into an IV port.
The tubing itself from her IV was spiked directly into a feeding pump bag. And the issue that allowed for that to happen was resolved before ENFit® was designed. Very quickly after her death, bags for enteral feeding and bags for IV solutions were changed so that this could never happen again.
ENFit® would not have saved Robin’s life. ENFit® products do NOT address where these misconnections were actually happening in the first place.
Given the financial motive that appears to be behind the actions of Tom Hancock, the original executive director of GEDSA, it kind of smacks of the idea that he decided he could make a lot of money as a result of the death of a woman he never even met if he used her story for his own financial gain. Nothing about this smells right.
Summarizing my thoughts
And so, if you want the short version of my thoughts on ENFit® , here they are. I think there are people that benefit from using ENFit® . And I won’t tell someone not to use it if they know all of this and find the benefits outweigh the risks in their situation.
However, all of us, whether we use ENFit® or not, need to join together as a unified voice to stand against any suggestion that ENFit® be mandated as the only option for everyone because there are those for whom ENFit® would be more risk than benefit.
It’s also important to see that this entire process was done extremely poorly. It speaks of the lack of value placed on tube-fed individuals. Tube-fed people deserve better than the amount of consideration and respect that they were given in the development of this product.
Their needs weren’t considered when the guy put in charge of the project from the start was someone who had already gravely violated the most basic rights of tube-fed people and shown a complete and total disregard for the health of children.
Their needs weren’t considered when it was seen that there was a legitimate concern as to whether this system would increase risk of infection and this clear fact was completely ignored. To this day, even the toothbrush method of cleaning has not been verified through peer-reviewed research to bring the bacterial load to an acceptable level.
I have heard there is a study coming out showing a toothbrush method does reduce bacterial load to an acceptable level, but that data isn’t available to anyone just yet for review. Are you getting this? We do not know for sure whether it is safe to use ENFit® , and yet it’s being used all over the world.
A proven system of adequate cleaning should have been developed BEFORE ENFit® was released. Not years later. Even if it turns out that this method is perfectly safe, the reality is that the tube-fed community had a system pushed upon them that wasn’t even tested thoroughly for safety.
I will never be ok with someone taking the tragic death of a mother and her baby and twisting it around to result in his own financial gain. ENFit® products do absolutely nothing to address the misconnection that resulted in her death. It is horrifying that her story has been manipulated in the way that it has been.
As a community, we need to demand better. We must insist that these terrible people who are using ill-designed research to promote their products stop being put in charge of anything relevant to the tube-fed community. We must demand proper research be done before products are used on our children and loved ones, treating them like guinea pigs.
Isn’t that what has already happened with enteral formula? Let’s just throw some stuff in a can, do absolutely no valid research on it whatsoever, and have kids fed it for years while they vomit, develop kidney problems, develop diabetes, experience reduced immune function, retain fluids, live with increased inflammation and more…
Enough is enough. The wrong guy was chosen for this in the first place, and it shows. We need people developing products for our tube-fed loved ones that have demonstrated that they actually care about the health of our loved ones rather than seeing our community as a means to line their pockets and nothing more, the health of tubies be damned.
I’ve had enough. Have you?

Wonderful and informative article! I personally have chosen not to use Enfit connections thus far. I had no idea about the history behind this newer product line and the associated complications that may come with its use. Thanks for all the information!
LikeLike
You did a masterful job of presenting the facts. As a supplier of silicone o-ring feeding syringes I learnt about Enfit from several in the blended food community. I then turned the info over to a relative who was successful in moving WGBH the Boston PBS station to run a piece about Enfit and resistance to it. Sanford Flach and his crew have been very vocal against Enfit – and they are right! For those looking for silicone o-ring feeding syringes that will work perfectly with blended diets – here are a few sources. Good luck to you all.
https://www.amazon.com/Reusable-Silicone-Syringe-Feeding-Garden/dp/B07BHWCFW4/ref=sr_1_7?crid=3OZW3NLWQT7P3&keywords=60cc+syringe+catheter+tip&qid=1550518440&s=gateway&sprefix=60cc%2Caps%2C402&sr=8-7
https://www.etsy.com/shop/SyringeShop?ref=seller-platform-mcnav
https://www.medcareproducts.com/O-Ring-Syringes-Non-sterile/productinfo/SBO/
LikeLike
Thanks for sharing!
LikeLike